The Effect of Electromagnetic Waves on Photosynthetic Pigments and Antioxidant Enzyme in Zea Mays L
Habibeh Zare1 and Sasan Mohsenzadeh1 *
DOI: http://dx.doi.org/10.12944/CWE.10.Special-Issue1.88
In order to investigate effects of electromagnetic wavelengths on content of antioxidant photosynthetic pigment in Zea mays L, petri dishes containing seeds soaked in water for 5 hours together with wet seeds were irradiated by electromagnetic waves (200 Gus intensity) every 8 hours and each time for about half an hour. After treatment the seeds were transferred to perlit and pitmass. 13 days old leaves were studied. Chemical analysis of acetone extract resulted from leaves, showed a decrease in the amount of chlorophyll a and b of under treatment samples compared to control. The difference between control and wet treatment samples was meaningful but there was not any significant difference between control and dry treatment samples. Under treatment samples revealed great increase in the amount of carotenoids and non-enzymatic antioxidants such as phenolic compounds, flavonoids and proline compared to control samples. In addition significant increase was observed in the activity of enzymatic antioxidants such as catalase, ascorbate peroxidase and superoxide dismutase related to wet treatment samples compare to control. However there was no significant difference between Off system treatment samples and control.
Copy the following to cite this article:
Zare H. and Mohsenzadeh S. The Effect of Electromagnetic Waves on Photosynthetic Pigments and Antioxidant Enzyme in Zea Mays L. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). DOI:http://dx.doi.org/10.12944/CWE.10.Special-Issue1.88
Copy the following to cite this URL:
Zare H. and Mohsenzadeh S. The Effect of Electromagnetic Waves on Photosynthetic Pigments and Antioxidant Enzyme in Zea Mays L. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). Available from: http://www.cwejournal.org/?p=9983
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-02-02 |
|---|---|
| Accepted: | 2015-03-30 |
Introduction
Biotic and abiotic stresses affect plant metabolism in different by induction of oxidative stress(Apel and Hirt, 2004). Electromagnetic waves transfer energy, so they may affect organisms including plants. Studies revealed that electromagnetic waves have effects on different aspects of plant life such as development and growth, breeding and function of plant cell structure. Depending on frequency of radiation and amount of transferred energy to the plant cell, plant responds differently. Electromagnetic waves with low frequency change the viability of seeds without any hard damage (Yao et al., 2006). Plants such as Zea mays L usually do not grow from seed and their proliferation is done by root division, stem propagation and shoots. Hence, we should prepare suitable conditions for germination. Priming is a method for seed promotion and also results in germination under stress conditions. Clearly in this method, first stages of germination including enzyme activation are passed by watered-seeds of course without any rootlet. Furthermore, electromagnetic waves shows induction activity and could speed up germination by affecting nucleus genes, increased metabolism and also by increasing enzyme activity and water absorbing
Electromagnetic waves also act as a stress inducer and change chemical content of stressed plant cells by oxidative stress in addition of increased reactive oxygen species (ROS). Plants produce different antioxidants compounds and store them to defuse stresses and increasing the resistance. Enhancement of carotenoids content is a defensive response of stressed plants. Carotenoids are known as protector of photosynthetic and chloroplast equipment in plants(Tevini et al., 1991). Increasing of carotenoids biosynthesis under stress may result in the reduction of wave destructive effects on chlorophyll pigments (IIao,1997). To improve defensive mechanisms, plants produce and aggregate phenols in their tissues and also store flavonoids compounds with different structural and biochemical properties use variety of mechanisms to scavenge free radicals (Shao et al.,2008). Production of proline is also another important adaptation of stressed plants. proline with different mechanisms involves in scavenging of cell ROS. Aggregation of proline in higher plants relates directly to the ROS enhancement (Maggio et al., 2002). Another defensive mechanism of stressed plants is increasing the activity of enzymatic antioxidants such as catalase, ascorbate peroxidase, and superoxide dismutase. Prostetic groups of these enzymes have an important role in scavenging free radicals(Alscher et al.,2002). As Zea mays L includes effective chemical compounds, the purpose of this study is to determine if stress of low intense electromagnetic waves could result in enhancement of plant without plant destructing.
Materials and Methods
In this study, Zea mays L seeds provided from Zare institute, 524 Hybride majar. Petri-dishes including seeds after sterilization were placed in H2O2 solution as an osmoprime. To prepare osmoprime solution was 100 ml distilled water. Seeds were placed in osmoprime solution for 5 hours and after priming, the solution was discarded and seeds were washed 3 times with distilled water and the remained water was taken by filtration paper then seeds were dried in lab temperature and prepared for next steps. Some seeds were soaked in 100 ml distilled water with 25 for 5h. In this study petri-dishes including wet seeds were placed horizontally on the surface of two Sheet of magnet in electromagnetic waves industrial system mad in Tayvan and every day and each time for 30 min were radiated by 20 mT electromagnetic waves. Then, seeds transferred to pot including perlit and pitmass compounds with pH=6 and 25 temperature. In 13 days old leaves of plants, photosynthetic pigments, amount of non-enzymatic antioxidants such as phenolic compounds, flavonoids, proline and activity of enzymatic antioxidants such as catalase, ascorbate peroxidase and superoxide dismutase was measured. Comparison between treatment and control was done 4 replicate on the basis of Duncan test by Spss 16 statistical software in p and plots were drawn using Excel.
Measurement of photosynthetic pigments amount: measurement of photosynthetic pigments such as chlorophyll a, b and also carotenoids sing acetone extract of leaf was done by Arnon and Lichtenthaler method. 0.2 gr fresh leaf tissues was grinned completely by 5ml 80% acetone, then resulting solution was filtered by Wattman filtration paper. Next, 5ml more acetone was added and absorbance of resulting solution was measured in 663, 646 and 480 nm using spectrophotometer (SPEKOL 1500). Concentration of photosynthetic pigments and carotenoids is measured by following equations.
Measurement of phenolic content: method of Mcdonald et.al was used to measure phenolic content. 0.5ml methanol extract with 5ml 1N folin-siocalto was mixed, then 4ml sodium carbonate 1M was added and distilled water added to make it 100cc. solution was placed in dark for 15 minutes and absorbance was measured in 765 nm. Galic acid was used to depict standard calibration curve.
Measurement of flavonoid content: evaluation of methanol extract to consider flavonoid content was done on the basis of Chang method. 0.5ml of methanol extract solution was mixed by 1.5cc methanol 95%, 0.1ml aluminium chloride 10% in methanol, 0.1cc potassium acetate 1M and 2.8ml deionized distilled water. After 30 minutes, absorbance was measured in 415nm. Galic acid was used to depict standard calibration curve.
Measurement of proline content: measurement of proline content was done by Bates et al, method. In this method 0.5gr fresh leaf was grinned with 10ml 3% solution of sulfusalcilic acid. 2ml was collected from homogenous mixture after filtration and addition of 2ml ninhydryn indicator plus 2ml Galic acid, closed tubes are placed in bain-marie with 100 tempretaure for 1h. After 1h, tubes are placed in ice to stop reaction. Then, 4ml toluene was added to each tube and stirred strongly. Absorbance of toluene phase was measured in 520 nm and amount of proline was achieved due to its standard curve.
Measurement of catalase enzyme activity: activity of catalase enzyme using estimating of reduction in H2O2 absorbance in 240nm was done on the basis of Dhindasa ant Motowe method. The mixture include 2.87ml of 50mM potassium phosphate pH=7 and 30μl of 15mM H2O2.then, 100mL enzymatic extraction was added to start reaction. Differences in absorption were measured. Amount of H2O2 in mixture was calculated using quenching constant ε=0.28mMol and A=εbc equation which shows extent of enzyme activity. A stands for absorbance, ε for quenching constant, c for H2O2 concentration and b for covet length (1cm). Enzyme activity is measured as total amount of existence protein (mg) per 100μl of extract in 1 minute.
Measurement of ascorbate peroxidase enzyme activity: measurement of peroxidase enzyme activity was done on the basis of Nakano and Asade method. The mixture reaction includes 50mM phosphate buffer with pH=7, 0.15M H2O2, 0.5mM ascorbate and 50μL enzyme extraction. Absorbance reduction in 290ηm was measured following ascorbate oxidation with beginning of enzymatic reaction. Constant was introduced equal to 2.8m and on the basis of A=εbc equation according to changes of absorption per minute.
Measurement of superoxide dismutase enzyme activity: method provided by Gianopolitis and Rics used to evaluate superoxide dismutase enzyme activity. Reaction mixture includes 2.5mL of 50mM potassium phosphate buffer with pH=7.8, 0.1mL 13 mM methionine, 0.1mL of 75mM nitrobluetetrazolium, 0.1 mL 2μM and 0.2mL enzyme extraction. Samples were exposed to light for 15 minutes and their absorbance were measured in 560ηm. In this work two control samples provided, first control sample without receiving light used as blank and second sample exposed to light for 15 minutes which nitroblutetrazolium reduction accomplishes completely under light due to enzyme presence. A unit of superoxide dismutase activity explains an amount of enzyme which results in 50% inhibition of light reduction of nitro blue tetrazolium.
Results
Measurement of photosynthetic pigments in control and treatment samples explained reduction in amount of chlorophyll pigments ( a and b) and increasing of carotenoids content of treatment samples (fig 1). According to the results obtained from statistical considerations, content of chlorophyll a, b in wet treatment compared to control decreased 7.45 and 18.35 respectively (fig 2) which is meaningful. Decreasing of chlorophll a, b content in off system treatment, obtained 2.69 and 4.85 respectively which is not meaningful (fig 3). Comparison of carotenoids content in both wet and dry treatment samples revealed a meaningful increase. The increase in wet treatment compared to control, and dry treatment compared to control calculated 18.13 and 11.18 respectively.
In accordance with documented results, electromagnetic waves lead to 85% increase of phenolic content and 52.73% increase in flavonoid content of dry treatment compared to control samples. This enhancement in dry treatment relative to control is calculated 29% and 31.42% for phenolic and flavonoid compounds respectively. There is a meaningful difference in both groups of treatment compared to control (fig 4). Statistical investigations showed that
As fig 5 shows, electromagnetic waves in stressed plants result in increasing of antioxidant enzyme activity. This increase in wet treatment relative to control for catalase, ascorbate peroxidase and superoxide dismutase enzymes calculated 55, 39.80 and 24.11 respectively which is meaningful. The same comparison was done between dry treatment and control which showed 11.89, 9.85 and 7.14 increase for catalase, ascorbate peroxidase and super oxide dismutase respectively but the difference was not meaningful.
 |
Figure 1: Device producing electromagnetic waves and the way it works Click here to View figure |
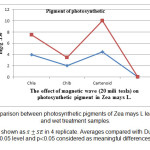 |
Figure 2: Comparison between photosynthetic pigments of Zea mays L leaf in control and wet treatment samples. Results are shown as in 4 replicate. Averages compared with Duncan test in 0.05 level and p0.05 considered as meaningful differences. |
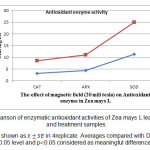 |
Figure 3: Comparison of enzymatic antioxidant activities of Zea mays L leaves in control and treatment samples. Results are shown as in 4replicate. Averages compared with Duncan test in 0.05 level and p0.05 considered as meaningful differences. Click here to View figure |
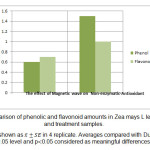 |
Figure 4: Comparison of phenolic and flavonoid amounts in Zea mays L leaf of control and treatment samples. Click here to View figure |
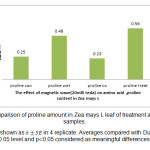 |
Figure 5: Comparison of proline amount in Zea mays L leaf of treatment and control samples. Click here to View figure |
Discussion
Plants detect stresses and respond to them. Stresses such as electromagnetic waves lead to induction of antioxidant production and changes in chemical content of plant. Increasing in amount of these compounds reveals activity of plant cells in order to neutralize or modulate effects of stress which its outcome is increased resistant of plant against stress. Plants like other organisms respond to stresses. On the basis of Hosseinio's theory and coworkers, plants using production of effective antioxidant compounds resist under different stress factors. One of the most important defensive mechanisms of plants to control free radicals is induction of some enzymatic and non-enzymatic antioxidants. Consideration revealed that a lot of metabolic processes of plants lead to production of ROS or free radicals but there are efficient mechanisms such as antioxidant mechanisms for scavenging (Blokina et al.,2003). In normal conditions, there's a balance between amount of Ros production and scavenging capacity by antioxidant defense. In contrast, amount of free radicals production in stressed conditions is more than their scavenging capacity. Collectively, variation of antioxidant defense capacity against these factors seems to be vital (Smirnoff, 1995).
In this study, decreasing of chlorophyll a, b content and increased carotenoid content of stressed plants was observed. In accordance with Jansen and coworker' theory, decreasing of chlorophyll may happen due to inhibition of related or by degradation of chlorophyll precursors under stress of electromagnetic waves. Caldwell and coworkers also reported that reason of chlorophyll reduction is chlorophyll degradation or negative effect on precursors for chlorophyll synthesis. Photosynthetic membrane could easily get damaged by absorbing high amount of energy using chloroplasts. There would be a protective mechanism if the energy is not trapped photochemically. Protective mechanism may be used as a valve which transfers extra energy. If this excited situation of chlorophyll is not suppressed quickly, so there would be possibility of reaction with molecular oxygen which results in excited form of oxygen (singlet oxygen) (IIao,1997). Carotenoids of chloroplasts protect this organelle against destruction effects of waves. These compounds are able to deactivate ROS produced in the stress by receiving high energy of short wavelengths and act as an antioxidant. Carotenoids work out by quick suppression of excited chlorophyll. Excited carotenoids are not capable to form singlet oxygen and returns to initial state by losing energy as heat(Yao et al., 2006). Increasing of non-enzymatic antioxidant including phenol, flavonoid and proline are some kind of defensive mechanisms which happens in plants with stresses of electromagnetic waves (consistent with our results). On the basis of Caldwell and coworker's opinion, electromagnetic waves cause induction of cinamic acid synthesis and induce phenilpropanoid pathway that provides effective antioxidantresistance against stress in higher plants. Limitation in transferring of photosynthetic electron of stress condition seems to be one reason for flavonoids synthesis induction. Inhibition of flavonoid biosynthesis pathway using inhibitors of phenyl alanine amunialyase synthesis lead to increased sensitivity of plants to waves. In accordance with Tevini and coworker's study, flavonoids are important antioxidant compounds which neutralize free radicals and also prevents from their extra production. Production and aggregation of flavonoids in vacuoles of stem and leaf epidermal cells cause wave absorbance and protects organelles like chloroplast against waves which reduces oxidative stress effects(Flint et al.,1985).Sakihama et.al reported that high aggregation of phenolic compounds inside epidermal cells and their cell wall, also fuzzes and vacuoles of epidermal cells prevents from damage in inner mesophilic cells and is used for photosynthetic carbon assimilation with improving defensive mechanisms. Sakihama et.al using mutants unable to produce phenolic compounds revealed importance of these compounds in rice against electromagnetic waves radiation. Proline also as an effective defensive compound protects cells against stress of waves and in stressed plants increases as a defensive or adaptation response (Maggio et al., 2002). Proline plays different roles in water balance, stability of proteins, enzymes and their 3D structure. Proline also is carbon and nitrogen source for growing after removing the stress. This compound cause reduction in ROS threat, scavenging of free hydroxyl radicals, quenching of singlet oxygen, adjustment of cell pH and adjustment of /NADPH. Proline has a role in stability of membrane by integrating with phospholoipid membrane and changing hydrated layer around biological molecules. This substance is also effective in decreasing of thylakoid membranes damage by scavenging and reduction of free radicals (Verbruggen and Hermans 2008). Proline as a storage source of carbon and nitrogen the resistance of plants against stress plays an important role in adaptation and protective responses of plants(Siripornadulsil et al., 2002). Investigations also explain a direct relation between proline aggregation in higher plants and ROS increasing. This increase also may be the reason for proline production from glutamic acid(Hare and Cress,1997). Increasing of catalase, ascorbate peroxidase and superoxide dismutase activity as antioxidant enzymes, consistent with present study, is another defensive mechanism which happens in plants under stress of electromagnetic waves. Alscher et.al reported that prostatic group of homoprotein enzymes has a key role in removing of plant free radicals. Superoxide as a main ROS and a poison compound in cells cause enzyme nature variation, lipid oxidation and DNA disruption. Superoxide dismutase as a metalo-enzyme changes superoxide ion into hydrogen peroxide and molecular oxygen which reduces its destructive effects. This enzyme act as antioxidant defense line against free radicals but produces hydrogen peroxide that is another poison element(Alscher et al., 2002). Resulted hydrogen peroxide under superoxide dismutase function and a lot of other natural mechanisms of cell by enzymes such as catalase, ascorbate peroxidase are controlled. Catalase using direct impact of these compounds, makes hydrogen peroxide a substrate and prevents from its destructive effects by changing this molecule to water and molecular oxygen(Hare and Cress,1997). peroxidase are also a great family of defensive enzymes which catalyzes redox reaction between hydrogen peroxide as an electron acceptor and a lot of substrates such as phenolic compounds, ascorbat acid, aromatic amines and cytochrome c. plant has been attacked by free radicals less when this enzyme is increased(jonsen et al., 1998). In stressed plants, ascorbate peroxidase is increased in cytosol, vacuole, chloroplasts and apoplastas which plays an important role in balancing of produced free radicals amount(Zao and Chang,2008). In this compound, ascorbate is an electron acceptor and reducer element which plays an important role in scavenging of hydrogen peroxide(Gill and Tuteja, 2010).
Conclusion
Priming of zea mays l seeds provides their germination and radiation of electromagnetic waves result in increased rate of germination. In zea mays l as a response to electromagnetic waves with low intensity, amount of non-enzymatic antioxidants was increased.
References
- Aladjadjiyan, A.2002.Study of the influence of magnetic field on some biological characteristics of Zea mays. Journal Central European Agriculture.3: 89-94.
- Alorainy, A. 2003. Recent research on mobile phones effects. The international conference on non-ionizing radiation at electromagnetic field and our health. Kula Lumpur, Analytical Biochemistry. 72: 248-254.
- Alscher, R.G. Ertuk, N. and Heath, L.S.(2002). Role of superoxide dismutase in controlling oxidative stress in plants.Journal of experimental botany. 327(9):1331-1341.
- Apel, K. nadHirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annual review of plant biology. 55(5):373-399.
- Arnon, DI. 1949. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiology. 24: 1-15.
- Ayrapetyan, G. 2006. The effect of EMF waves on barley seed hydration and germination potential. Journal of Electromagnetic Waves and Applications 4: 65-76.
- Bates, LS., Walderen, RD. and Taere, ID. 1973. Rapid determination of free proline for water stress studies. Plant and Soil 39: 205-207.
- Belyavskaya, N. Biological effects due to weak magnetic field of plants. Advances in Space Research.34: 1566-1574.
- Cakmak, T., Dumlupinar, R. and Erdal, S. 2009. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics. 31: 120-129.
- Blokina, O., virulainen, E. and fagersted, K. (2003). Antioxidant, oxidative damage and oxygen deprivation stress. Annals of botany. 91(4): 179-194.
- Caldwell, M.M, Robberechet, R. and Flint, S.D. (1983). Internal filters: prospects for UV acclimation in higher plants. Physiological plantarum. 58(8)445-450.
- Chang, C., Yang, M., Wen H. and Chern, J. (2002).Stimation of toyalflavonoidcontent in propolis by two complementary colorimetric methods.Journal of food and drug analysis. 10(1)178-182.
- Dhindsa, R.R. and Motowe, W. (1981).Drough tolerance in two mosses: correlation with enzymatic defense against lipid peroxidation. Journal of experimental botany. 32(4): 79-91.
- Flint, S.D., Jordan, P.W. and Caldwell, M.M. (1985). Plant protective responses to enhancedUV-B radiation under field condition: leaf optical properties and photosynthesis. Journalof photochemistry and photobiology. 41(4):95-99.
- Gao, O., and Zhang, L. (2008). Ultraviolet-B-induced oxidative stress and antioxidabt defense system responses in ascorbate deficient vtc1 mutants of Arabidopsis thaliana. Journal of plant physiology. 22(4): 138-148.
- Gianopolitis, C. and Ries, S.K. (1977).Superoxide dismutase occurrence in higher plants.Journal of plant physiology. 59(2):309-314.
- Gill, S.S. and tuteja, N. (2010).Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.Plant physiology and biochemistry. 132(3):909-930.
- Hare, P.D. and cress, W.A. (1997).Metabolic implications of stress-induced accumulation in plants.Plant growth regulation. 21(2):79-103.
- Hosseinisarghein, S., Carapetian, J. and Khara, J. (2011).The effects of Uv radiation on some structural and ultra-structural parameters in peper. Journal of Turkish studies. 35(8): 69-77.
- IIao, B.A. (1997). The effects of ultraviolet-B- radiation and co2 on growth and photosynthesis of tomato.Canadian journal of botany. 75(2): 213-219.
- Jansen, M., gaba, V. and Greenberg, B.M. (1998). Higher plants and UV-B radiation: balancing damage, repair and accumulation. Trends in plant science. 3(1): 131-135.
- Lichtenthaler, H.K. (1987). Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Method of nzymology. 148(4): 350-382.
- Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant cell physil. 22(1): 867-880.
- Maggio, A., Miyazaki, S. and Veronese P. (2002).Does proline accumulation play an active role in stress induced growth reduction? The plant journal. 31(5): 699-712.
- McDonald, S., Prenzler, P.D, Autolovich, M. and Robards, K. (2001). Phenolic content and antioxidant activity of olive extract food chemistry. 73(9): 73-84.
- Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in plant science. 7(1): 405-410.
- Sakihama, Y., Cohen, M.F, Grace, S.C. and Yamaski, H> (2002). Plant phenolic antioxidant and perooxidant activities: phenolic induced oxidative damage mediated by metal in plants. Toxicology. 177(9): 67-80.
- Shao, H.B., Chu, L.Y., ZH, L.U. and Kang, C.M (2008).Primary antioxidant free radical scavenging and redox signaling pathway in higher plant cells.International journal of biological science. 4(1): 8-14.
- Siripornadulsil, S., Traina, S., Vrma, D.P. and sayer, R.T. (2002).Molecular mechanism of proline-mediated tolerance to toxic heavy metals in transgenic microalgae.Plant cell. 14(7): 217-243.
- Smirnoff, N. (1995). Antioxidant systems and plant response to the environment; in environment and plant metabolism.Bioscientific publisher, oxford, united kingdom. 111(2)217-243.
- Tevini, M., Braun, J. and Fieser, G. (1991).The productive function of the epidermal layer of rye seedling against ultraviolet-B radiation.Journal of photochemistry and photobiology.Verbruggen, N., Hermans, C. (2008). Proline accumulation in plant: a review. Amino acids. 35(2): 753-759.
- Yao, Y., Xuana, Z. and Li, Y. (2006).Effect of ultraviolet-B radiation on crop growth, development, yield and leaf pigment concentration of tartarybucj wheat uder field conditions.European journal of agronomy. 25(7): 215-222.






